ACID & BASES
Theories of Acid and Base
Three important theories are
- 1. Arrhenius theory
- 2. Lowry and Bronsted theory
- 3. Lewis theory
1. Arrhenius Theory (Dissociation concept)
i. Acid is a substance, dissociates to give hydrogen ions (H+) in water.
eg: HCl
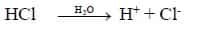
ii. Base is substance, dissociates to give hydroxide ions (OH-) in water.
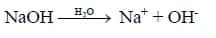
2. Lowry-Bronsted Theory (Proton Concept)
According to them
i. Acids are called as proton donors which donates protons in solution to any other substance
ii. Bases are called as proton acceptors which accept protons in solution from any other substance.
NH3 +HCl → NH+4Cl–
In the above reaction, HCl donates a proton and ammonia accepts that proton forming ammonium chloride.
So, according to this theory HCl is an acid and ammonia is a base.
3. Lewis Theory (Electron Concept)
Based on this theory, acids are called as electron acceptors which accept a lone pair of electrons. Bases are called as electron donors which donate a lone pair of electrons in solution.
Eg : H+ + NH3 → NH4+
In the above reaction, proton (H+) accepts one electron pair from NH3 and is, therefore, an acid, whereas NH3 molecule donates an electron pair is a base.
BORIC ACID
M.F. H3BO3;
Syn : Ortho Boric Acid
Preparation:
(i) Laboratory Method
Adding a mixture of concentrated sulphuric acid and water to a boiling solution of borax, the solution is allowed to cool. The boric acid is filtered and then washed until they become free from sulphate ions.
Na2B4O7 + H2SO4 + 5H2O → Na2SO4 + 4H3BO3 (
(ii) Commercial Method or Industrial Method
It is prepared commercially by decomposing certain naturally occurring borates such as colemanite, resonite, borax, etc. eg. Cole manite is suspended in boiling water then sulphur-di-oxide gas is passed through the suspension to liberate boric acid.
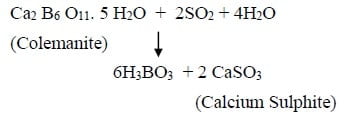
Physical Properties
- i. White odourless, crystalline powder, soft to touch.
- ii. Slightly acidic to taste.
- iii. Freely soluble in boiling water, boiling alcohol and glycerin.
Chemical Properties
i. Boric acid is a weak acid. On heating to 100°C loses one molecule of water to give meta boric acid.
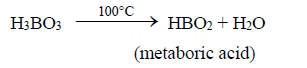
ii. Upon further heating to 160°C, further loss of water from metaboric acid to tetra boric acid.
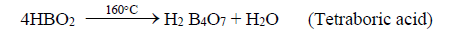
iii. On heating tetra boric acid produces the boric acid anhydride, boron trioxide
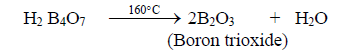
iv. One molecule of acid reacts with only one mole of sodium hydroxide.
NaOH + H3BO3 → NaBO2 + 2H2O
Assay
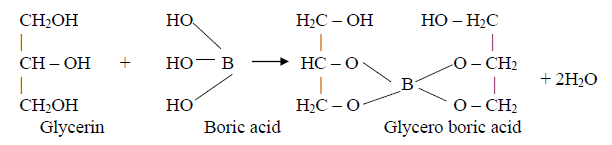
Boric acid is assayed by titrimetric method. It is a very weak acid, hence it cannot be titrated directly with a base to a sharp endpoint. It is dissolved in a mixture of water and glycerin and it is made as strong acid i.e., Glyceroboric acid and then it can be titrated with sodium hydroxide to phenolphthalein as an indicator. The endpoint is the appearance of permanent pale pink color.
Storage : Well closed container
Use : Anti infective
HYDROCHLORIC ACID
M.F. : HCl
Synonyms: Muriatic acid, Acidum hydrochloricum, Spirit of Salt.
It is a solution of hydrogen chloride (HCl)gas in water containing not less than 35.0% & not more than 38% by weight of HCl.
Preparation
(i) It is manufactured by the action of sulphuric acid on sodium chloride (common salt)
2NaCl + H2SO4 → Na2SO4 + 2HCl
(ii) Hydrogen and chloride gases (obtained from Electrolysis of sodium chloride solution) are combined to give hydrogen chloride gas which is dissolved in water to yield hydrochloric acid.
H2 + Cl2 → 2HCl
Properties (Physical & Chemical)
- It is a colourless, fuming liquid having a pungent odour.
- It gives white precipitate with silver nitrate.
AgNO3 + HCl → AgCl (ppt) + HNO3
Identification Test
i. When it is added to KMnO4, chlorine gas is liberated.
Assay : Titremetric method (acid-base titration)
It is assayed by titration with sodium hydroxide using methyl orange as an indicator.
NaOH + HCl → NaCl + H2O
Storage: It should be stored in well closed non-metallic container.
Use : as solvent, catalyst and also as an acidifier.
STRONG AMMONIA SOLUTION
M.F. : NH3
Syn: Liquior ammonia fortis/ Ammonium solution
STRONG AMMONIA SOLUTION : Contanis 27.0-30.0% w/w of NH3
Preparation : Laboratory Method
Heating ammonium chloride with calcium hydroxide.
2 NH4Cl + Ca(OH)2 → 2NH3(gas) + CaCl2 +2 H2O
Commercial or Industrial Method
Haber Synthesis
Hydrogen and nitrogen gases are combined to give ammonia gas.
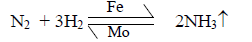
Physical Properties
i. It is clear, colourless, transparent liquid having characteristic strong pungent odour.
ii. Highly soluble in water.
Chemical Properties
i. Ammonia is able to reduce potassium permanganate to MnO2.
2NH3 + 2KMnO4 → 2KOH + 2MnO2 + 2H2O + N2(gas)
ii. Ammonia is a strong base. Therefore it reacts with acids to form salts.
NH3 + HCl → NH4Cl
Identification
When a glass rod dipped in HCl is brought near the surface of the solution, white fumes will be produced.
Assay : Back Titration Method
Weighed amount of ammonia is added to the excess of sulphuric acid. The excess or unreacted sulphuric acid is back titrated by titration with sodium hydroxide solution using methyl red as indicator.
2NH3 + H2SO4 → (NH4)2SO4
H2SO4 + 2NaOH → Na2SO4 + 2H2O
Storage: It is stored in well closed amber coloured container having a rubber stopper in cool place.
Use : Laboratory reagent, antacid, stimulant, counter irritant.
CALCIUM HYDROXIDE
M.F.: Ca(OH)2, Mol. wt; 74.10
Syn.: Slaked lime
Preparation Slaking Process
Spraying water on quicklime
CaO (quicklime) + H2O → Ca(OH)2
Properties:
- i. It is a soft white powder, having a slightly bitter taste. ii. It is soluble in water and in alcohol.
- iii. On exposure to air, it absorbs atmospheric CO2 and forms CaCO3.
- iv. When it is heated strongly, it loses water and is converted to CaO.
Ca(OH)2 → CaO + H2O
A clear saturated solution of calcium hydroxide in water is called lime water.
Assay : Complexometric Method
A weighed amount of calcium hydroxide is mixed with sufficient amount of dilute HCl. A known volume of the above solution is mixed with 15 ml of NaOH solution and 3 ml of napthol green are added titrated with disodium ethylene diamine tetra acetate (EDTA) to the deep blue colour end point using murexide as an indicator.
Storage
It should be stored in well-closed container, protected from moisture and carbon dioxide.
Use :(i) antacid, (ii) as an astringent (iii) Topically as a protective.
SODIUM HYDROXIDE
M.F: NaOH
Syn: Caustic Soda.
Preparation : Industrial method
Soda Lime Process
1. Sodium carbonate is heated with milk of lime.
Na2CO3 + Ca (OH)2 → CaCO3(ppt) + 2NaOH
Properties (Physical & Chemical)
- It occurs in the form of flakes, sticks and pellets.
- It is very deliquescent.
- It is strongly alkaline and corrosive.
- It is soluble in water, alcohol and in glycerin with the production of heat.
- It absorbs carbon-di-oxide and gets partially converted into sodium carbonate.
H2O + CO2 → H2CO3
2NaOH + H2CO3 → Na2CO3 + 2H2O
Assay
As sodium hydroxide absorbs CO2, it contains a little sodium carbonate. Hence it is assayed by two steps (for the estimation of total alkali and sodium carbonate in the sample).
1st Step
Weighed sample is dissolved in CO2 free water and titrated with sulphuric acid using Phenolphthalein solution as an indicator, until the pink colour of the solution in discharged.
2 NaOH + H2SO4 → Na2SO4 + 2H2O
2 Na2CO3 + H2SO4 → Na2SO4 + 2NaHCO3
As per the above equations, all the NaOH has been neutralized by H2SO4 and Na2CO3 is converted into NaHCO3. This step is called as half neutralization of sodium carbonate.
IInd Step
To the above solution, methyl orange is added and the titration is continued until a permanent pink colour is produced.
2NaHCO3 + H2SO4 → Na2SO4 + H2O + CO2
Storage: It must be stored in tightly closed container.
Use :
- Laboratory reagent
- to remove wart (as caustic agent) & Veterinary Disinfectant-2.5 % solution in glycerol
- Pharmaceutical Alkalising agent for adjusting pH
BUFFERS
Such solutions for which the pH will not be changed when a small amount of acid or base is added. Buffer solutions consists of a mixture of weak acid and salt of its strong base or weak base, and the salt of its strong acid.
A buffer is a solution composed of a weak acid (HA) and the salt of that weak acid (i.e., the conjugate base, A-), or a weak base (B) and the salt of that weak base (i.e., the conjugate acid BH+).
When a strong acid is added to a buffer system, the “basic” component of the buffer (A– ) reacts with it in an attempt to
“neutralize” it. When a strong base is added, the “acidic” component (HA) of the buffer reacts with it to neutralize it.
Buffers are most effective in the pH range of ± 1 unit to either side of the pKa, or the pOH (i.e., 14-pH) range ± 1 unit to either side of the pKb of the desired system. Closer the concentrations of the weak acid and its conjugate weak base salt or the weak base and its conjugatem weak acid salt are to one another, the better the chance of achieving greater buffering effect.
Types of buffers:
1. Acidic buffer solutions:
Acidic buffers consist of a weak acid in equilibrium with its salt (conjugate *base) in an aqueous environment or Mixture of weak acid and the salt is called as above.
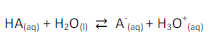
e.g., CH3COOH + CH3COONa
2.Basic Buffer
Mixture weak base and the salt (equimolar amounts of the weak base and its conjugate acid are used)
eg : NH4OH and NH4Cl
Buffer Action
The resistance possessed by buffers to change in pH is defined as buffer action.
Standard Buffer Solutions
Standard buffer solutions are solutions of standard pH.
Official Buffers
The buffer solutions recommended by pharmacopoeia are called as official buffers.
e.g.
1. Acid phthalate buffer : It contains potassium hydrogen phthalate and hydro chloric acid.
2. Alkaline borate buffer : It contains boric acid, potassium chloride and sodium hydroxide.
3. Phosphate buffer : It contains potassium dihydrogen phosphate and sodium hydroxide.
4. Acetate Buffer : (pH 2.8) : It contains sodium acetate and glacial acetic acid.
Uses of Buffers
Buffers are used to maintain the pH value.
- i. pH of the blood is maintained by the buffer system present in our body.
- ii. Solubility of the many compounds can be controlled by providing suitable pH.
- iii. It provides a particular pH for maximum enzyme activity. Hence it is used in the assay of enzyme activity.
- iv. They are very much useful in the estimation of metallic salts by complexometric titrations since the EDTA – metal complex is more stable at a particular pH.
- v. Certain pharmaceutical preparations are stabilized by adding suitable buffers.










