Radiopharmaceutical
Radiopharmaceuticals are a group of pharmaceutical drugs/medicinal formulation containing radioisotopes which are used as diagnostics & therapeutic agents.
Type of Radiopharmaceuticals products
Radiopharmaceuticals can be divided into four categories:
1. Radiopharmaceutical preparation
A radiopharmaceutical preparation is a medicinal product in a ready-to-use form suitable for human use that contains a radionuclide. The radionuclide is integral to the medicinal application of the preparation, making it appropriate for one or more diagnostic or therapeutic applications.
2. Radionuclide generator
A system in which a daughter radionuclide (short half-life) is separated by elution or by other means from a parent radionuclide (long half-life) and later used for production of a radiopharmaceutical preparation.
3. Radiopharmaceutical precursor
A radionuclide produced for the radio-labelling process with a resultant radiopharmaceutical preparation.
4. Kit for radiopharmaceutical preparation
In general a vial containing the non-radionuclide components of a radiopharmaceutical preparation , usually in the form of a sterilized, validated product to which the appropriate radionuclide is added or in which the appropriate radionuclide is diluted before medical use. In most cases the kit is a multidose vial and production of the radiopharmaceutical preparation may require additional steps such as boiling, heating, filtration and buffering. Radiopharmaceutical preparations derived from kits are normally intended for use within 12 hours of preparation.
The facilities and procedures for the production, use, and storage of radiopharmaceuticals are subject to licensing by national and/or regional authorities. This licensing includes compliance both with regulations governing pharmaceutical preparations and with those governing radioactive materials. Additional regulations may apply for issues such as transportation or dispensing of radiopharmaceuticals.
Shelf-life
The shelf-life (expiry period) of a radiopharmaceutical preparation depends primarily on the physical half-life of the radioisotope, the radiochemical stability and the content of longer-lived radionuclidic impurities in the preparation under consideration.
Manufacturing process for radiopharmaceutical preparations
The manufacturing process for radiopharmaceutical preparations should meet the requirements of Good Manufacturing Practice (GMP).
Manufacturing of radionuclides/ radiopharmaceutical preparations are include
a. Nuclear fission
Nuclides with high atomic number are fissionable and a common reaction is the fission of uranium-235 by neutrons in a nuclear reactor. For example, iodine-131, molybdenum-99 and xenon-133 can be produced in this way. Radionuclides from such a process must be carefully controlled in order to minimize the radionuclidic impurities.
b. Charged particle bombardment
Radionuclides may be produced by bombarding target materials with charged particles in particle accelarators such as cyclotrons.
c. Neutron bombardment
Radionuclides may be produced by bombarding target materials with neutrons in nuclear reactors . The desired nuclear reaction will be influenced by the energy of the incident particle and by the isotopic composition and purity of the target material.
d. Radionuclide generator systems
Radionuclides of short half-life may be produced by means of a radionuclide generator system involving separation of the daughter radionuclide from a long-lived parent by chemical or physical separation.
e. Starting materials (including excipients)
In the manufacture of radiopharmaceutical preparations, measures are taken to ensure that all ingredients are of appropriate quality, including those starting materials, such as precursors for synthesis.
f. Target materials
The composition and purity of the target material and the nature and energy of the incident particle will determine the relative percentages of the principal radionuclide and other potential radionuclides (radionuclidic impurities) and thus ultimately the radionuclidic purity.
Each batch of target material must be tested and validated in special production runs before its use in routine radionuclide production and manufacture of the preparation, to
ensure that under specified conditions, the target yields a radionuclide in the desired quantity and quality.
g. Carriers
A carrier, in the form of inactive material, chemically similar to the radionuclide, may be added during processing and dispensing of a radiopharmaceutical preparation to permit ready handling. It will be necessary to add carrier to enhance chemical, physical or biological properties of the radiopharmaceutical preparation. A carrier must not cause undesirable physiological effects.
h. Carrier-free
Radioactive preparations in which no carrier is added during the manufacture or processing may be referred to as carrier-free preparation. The designation no-carrier-added is sometimes used to indicate that no dilution of the specific activity has taken place by design, although carrier may be present due to the natural presence of a non-radioactive element or compound accumulated during the production of the radionuclide or preparation of the compound in question.
Production of Radiopharmaceutical preparation
Radiopharmaceutical preparations may contain the excipients permitted by the general monograph for the relevant the dosage form & Pharmacopoeia.
Sterilization Radiopharmaceutical preparations intended for parenteral administration are sterilized by a suitable method.
Addition of antimicrobial preservatives
Radiopharmaceutical injections are commonly supplied in multi-dose containers. The nature of the antimicrobial preservative, if present, is stated on the label or, where applicable, that no antimicrobial preservative is present.
Radiopharmaceutical injections for which the shelf-life is greater than one day and that do contain an antimicrobial preservative may be supplied in multi-dose containers. After aseptic withdrawal of the first dose, the container should be stored at a temperature between 2° and 8°C and the contents used within 7 days.
Identity tests
Tests for identity of the radionuclide are included in the individual monographs for radiopharmaceutical preparations. The radionuclide is generally identified by its half-life
or by the nature and energy of its radiation or by both as stated in the monograph.
Half-life measurement
The preparation to be tested should be tested after appropriate dilution to avoid dead time losses using an ionization chamber, a Geiger-Muller counter, a scintillation counter or a semiconductor detector. The activity must be sufficiently high to allow detection during several estimated half-lives. The measured half-life should not deviate by more than 5% from the half-life stated in the individual monograph.
Radionuclidic purity
Requirements for radionuclidic purity are specified in two ways:
- By expression of a minimum level of radionuclidic purity.
- By expression of maximum levels of specific radionuclide impurities in the
individual monographs.
Radiochemical purity
Radiochemical purity is assessed by a variety of analytical techniques such as liquid chromatography, paper chromatography, thin-layer chromatography and electrophoresis.
Chemical Purity
Chemical purity refers to the proportion of the preparation that is in the specified chemical form regardless of the presence of radioactivity; it may be determined by accepted methods of analysis.
pH
When required, measure the pH of non-radioactive solutions.
Sterility
radiopharmaceuticals to be sterile is subjected to Test for sterility.
Bacterial endotoxins/ pyrogens
Where appropriate, a radiopharmaceutical preparation requires compliance Test for bacterial endotoxins.
Labelling
Every radiopharmaceutical preparation must comply with the labelling requirements established under Good Manufacturing Practice.
Isotope & Radioisotopes
Atomic Structure:
All the matters are made up of atoms. Bonding of atoms of same or different elements forms the molecule. An atom consist of nucleus and electrons revolving around the nucleus in different orbitals. The electrons have negative charge. The nucleus contains positively charged protons and neutral neutrons. The protons and neutrons have definite mass. An atom consist of an equal number of protons and electrons, then the atom is electrically neutral, for that atom the total electrical charge is zero.
Atomic Number:
Number of protons (Z) which is equal to the number of electrons in a neutral atom.
Mass Number (A):
Total number of protons (Z) and neutrons (N).
So Mass number (A) = Z + N
To represent an atom with all the above details it is written as below:
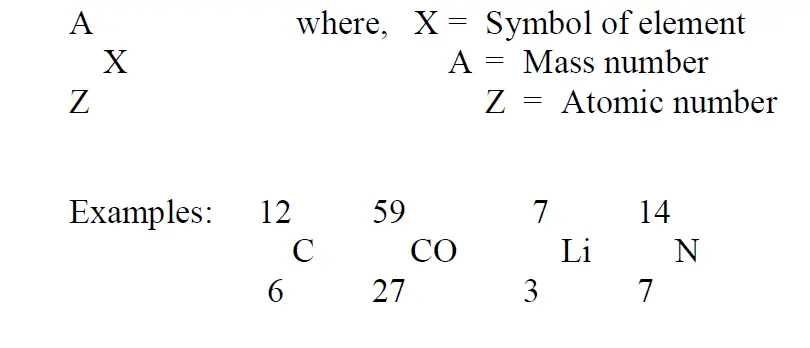
Isotopes
Atoms having the same atomic number but different mass numbers.
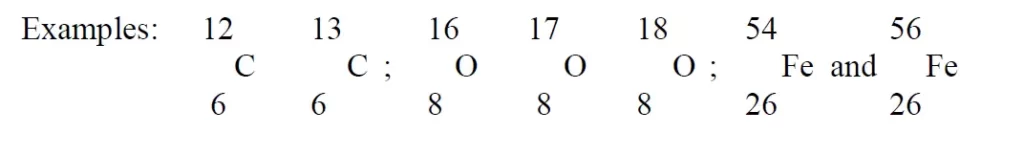
Types of Isotopes:
There are two types of Isotopes:
Stable Isotope
- Stable so do not emit radiation
- Carbon 12, chlorine 35, Hydrogen 1 (protium), Hydrogen 2 (Deuterium)
Radioactive Isotopes
- Unstable so they emit radiations
- Occurs naturally (Uranium, Radium etc) or may produce artificially.
- The phenomenon of emitting radiation by these isotopes is known as radioactivity and such isotopes are called radioactive isotopes.
Isobars:
Atoms having the same mass number but different atomic number are called isobars.
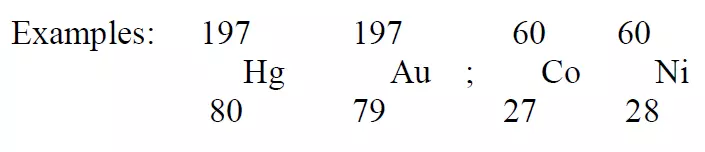
Radio Activity
Some elements are able to emit certain invisible rays which affect a photographic plate in the dark. These rays are also able to penetrate solid matter, ionise gases and produce luminescence in substance like zinc sulphate and barium salts. Such substances are called radio-active and the property is called radio-activity. Naturally, radio-active elements are uranium, radium, thorium etc.
A nucleus of an atom having same number of neutrons and protons are stable that is neutron to proton ratio is 1:1 and these atoms does not emit any type of radiation. A nucleus in which the number of neutron is different than the number of protons that is neutron to proton ratio is not equal and these atoms emits radiation. While emitting radiation the parent atom undergoes transformation and produces another daughter atom, and emitted radiation may be alpha rays (-particle) Beeta rays (-particles) and gamma radiation.
The Properties of radiations
Radiations emitted by atoms are of two types. They are particulate and electromagnetic. The most important particulate radiations are the alpha (α) and beta (β) radiations emitted by disintegrating atoms of radio nuclides.
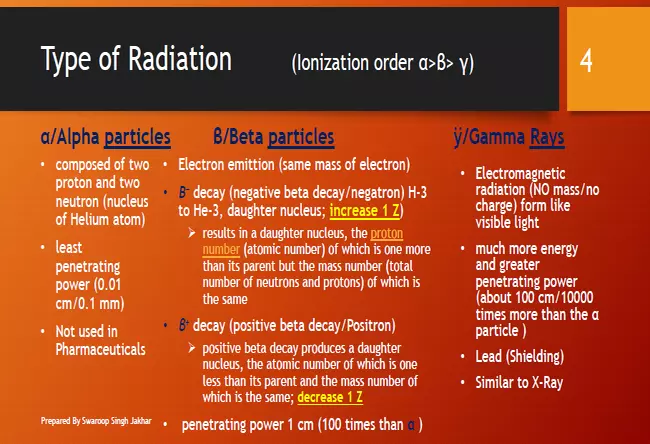
Alpha Particles (α)
- The alpha particles are positively charged.
- When radio active element emits alpha particles the resulting nucleus of the atom will have two positive charges less than the original nucleus. It can be illustrated by the decay of Radium nucleus to give randon nucleus.
- They are very much heavier than β particles.
- They have less penetrating power.
- They have very great ionising powers (high specific ionisation).
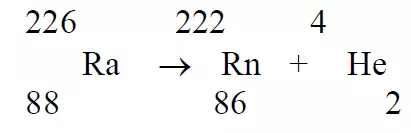
Beta Particles (β)
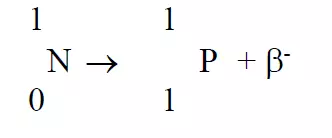
- Beta particles are negatively charged.
- They are heavier than α particles.
- They have more penetrating power than α particles as it can penetrate tissues.
- They are relatively less ionizing powers than α particles.
- β particles sometimes referred as negatrons which are emitted by unstable nuclei in which the neutron / proton ratio exceeds the stability limit and in such case neutrons are transformed protons with beta emission.
Gamma Radiations
- Gamma radiations are electromagnetic radiation similar to light and X-rays but of higher energy.
- These radiations do not have any charge and thus are not affected by electric or magnetic field.
- They are very short wave length resembling X-rays and travel with the velocity of light.
- They have poor ionizing power and very high penetrating power.
- They can interact with molecules and atoms in specific media and can produce ions and free radicals.
Biological Effects of Radiations
- These chemical species can alter the local pH resulting in the production of other toxic compounds.
- Life period may be shortening if a person is exposed to a smaller dose of radiation.
- It may cause sterility and it also may induce cancer.
- These radiations may alter the DNA leading to destruction of tissues or organs.
- Radiation may cause anaemia and decreases the blood cells.
Measurement of Radioactivity
The different kinds of particles and rays produced during the disintegration of a radioactive material leave number of ions along their paths. These ions are normally detected and measured. Measurement of radioactivity is based on the following properties of radiation.
- Radiation to cause ionization of gases.
- Radiation to cause flash of light.
- Radiation to cause chemical change
The different devices used to measure the radiations are

- Ionization chamber
- Proportional counters
- The Geiger Muller counter
- Scintillation counters
- Semi conductor detectors
- Photographic plate method
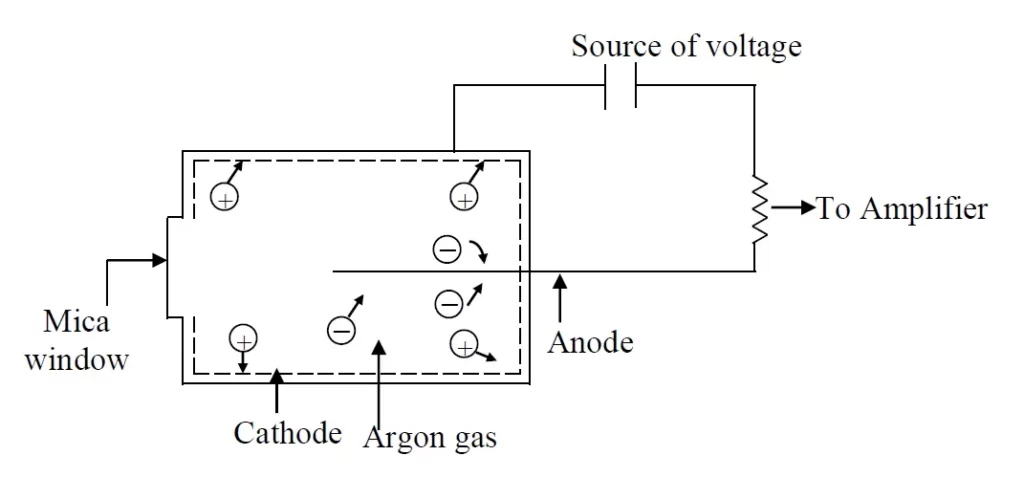
Geiger-Muller Counter
This is the most popular radiation detector because, it does not need a well-stabilized high voltage supply. It is most frequently used for detecting β- particles.
It contains a central wire anode, usually made of tungsten, and a cathode, silver, is coated in the inner side of the cylinder. The space between the electrodes (anode and cathode) contains a gas, which is usually an argon at a pressure of a few mm of mercury. The chamber is filled with argon gas and if the radiation is passed through the mica window of the chamber, the gas is ionized and there is a flow of current each particles of radiation causes a brief flow or pulses of current which is recorded by a device known as scalar which shows the total number of pulses.
Biological Half Life (t½ )
It is defined as the time in which the amount of radio nuclide decays (decomposes) to half of its initial value. It is related to decay constant λ.
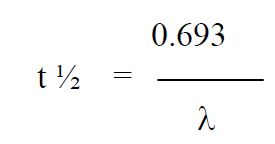
General Storage and Precautions of Radio Active Compounds
- Great care must be taken in the use and storage of radio active materials.
- For protecting and radiations, requires thick glass containers or Perspex containers are necessary.
- For protection radiations requires havier lead shielding or containers are necessary.
- Radioactive materials should never be touched with hand, it should be handled by means of forceps or suitable instruments.
- Smoking, drinking or eating activities are to be avoided in the laboratory where the radioactive materials are handled.
- Sufficient protective clothing or shielding must be used while handling the materials.
- Radioactive materials should be kept in suitable labeled containers and kept preferably in a remote corner.
TERMINOLOGY
Nuclide
A unique atom characterized by its atomic number (number of protons in the nucleus) and its atomic mass number (total number of neutrons and protons in the nucleus) and having stability such that its lifetime is measurable. All atoms sharing the same atomic number are the same element.
Isotopes
Atoms of the same element with different atomic mass numbers are called isotopes.
Radioactivity
The property of certain nuclides of emitting radiation by the spontaneous transformation of their nuclei into those of other nuclides.
NOTE. The term “disintegration” is widely used as an alternative to the term “transformation”. Transformation is preferred as it includes, without semantic difficulties, those processes in which no particles are emitted from the nucleus.
Radioactive decay
The property of unstable nuclides during which they undergo a spontaneous transformation within the nucleus. This change results in the emission of energetic particles or electromagnetic energy from the atoms and the production of an altered nucleus. This process is known as Radioactive decay.
Units of radioactivity
The activity of a quantity of radioactive material is expressed in terms of the number of spontaneous nuclear transformations taking place in unit time. The SI unit of activity is the becquerel (Bq), a special name for the reciprocal second (s-1). The expression of activity in terms of the becquerel therefore indicates the number of transformations per second. The historical/old unit of activity is the curie. The curie (Ci) is equivalent to 3.7 x 1010 Bq.
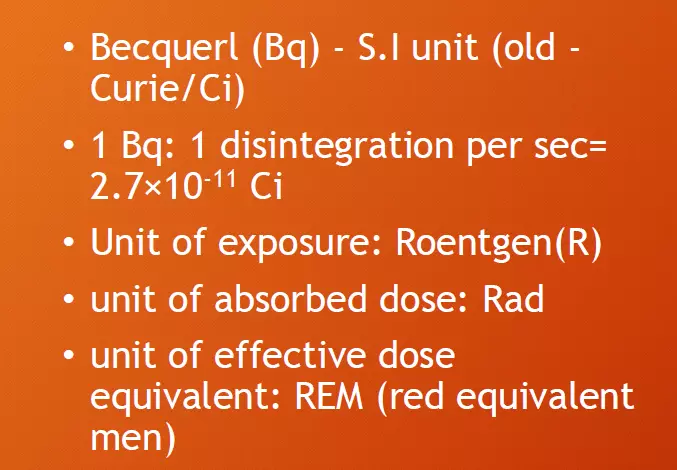
Units of Exposure: Roentgen (R)
Units of effective dose equivalent: REM (Red Equivalent men)
Units of absorbed dose: Rad
Also Read…
Official preparations of Radio Pharmaceuticals and their Applications
Join Our WhatsApp Group to receive the latest updates like Pharma Job notifications, study materials, admission alerts, Pharma News, etc
Join Our Telegram Group to receive the latest updates like Pharma Job notifications, study materials, admission alerts, Pharma News, etc
Join Our Telegram Group to Download Free Books & Notes, Previous papers for D.Pharm, B.Pharm, M.Pharm, Drug Inspector & GPAT……….










