Apparatus Requirements:
(a) Glassware: Nesslers’ cylinder, measuring cylinder and glass rod.
(b) Chemicals: Potassium sulphate, test substance, hydrochloric acid, barium sulphate reagent and distilled water.
Principle of Limit test for sulphate
Limit test of sulphate is based on the reaction of soluble sulphate with barium chloride in presence of dilute hydrochloric acid to form barium sulphate which appears as solid particles (turbidity) in the solution.
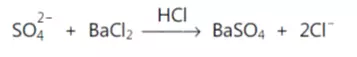
Due to the formation of precipitates, the solution appears turbid and the extent of turbidity depends on the amount of sulphates present. If the turbidity produced by the test is less than that of standard, it means that the sample contains sulphates within the prescribed limits.
Reagent Preparations:
Barium Sulphate Reagent:
Dissolve 12 g of barium chloride (BaCl2.2H2O) in 1000 ml water to make 0.05 M barium chloride solution. To 15 ml of the prepared solution, add 55 ml water, 20 ml alcohol, 5 ml of 0.0181%w/v potassium sulphate (K2SO4) solution and makeup the volume upto 100 ml.
Standard Potassium Sulphate Solution:
Accurately weigh 0.1089 g of K2SO4 was taken and the volume was made up to 100 ml with water.
Test sample:
(i) Sodium chloride: Dissolve 2 g of sodium chloride in 15 ml of water.
(ii) Sodium bicarbonate: Dissolve 2 g of sodium bicarbonate in 15 ml water
Procedure for limit test for Sulphate
Take two 50 ml Nessler cylinders. Label one as “Test” and the other as “Standard”
| Test | Standard | |
|---|---|---|
| Take 1 ml of 25% w/v solution of barium chloride in Nesslers’cylinder. | Take 1 ml of 25%w/v solution of barium chloride in Nesslers’ cylinder. | The barium ions produced, react with sulphates to form Precipitates (opalescence) of barium sulphate. |
| Add 1.5 ml of ethanolic sulphate standard solution (10 ppm of SO2− 4 ). | Add 1.5 ml of ethanolic sulphate standard solution (10 ppm of SO2− 4 ). | The ethanolic solution of potassium sulphate increases the sensitivity of test and alcohol prevents the super saturation of barium sulphate precipitates. |
| Add 15 ml of test sample solution prepared as directed in individual monograph. | Add 15 ml of standard potassium sulphate solution prepared as directed in individual monograph. | It gives total sulphate ion present in the sample |
| Add 0.15 ml of 5 M acetic acid. | Add 0.15 ml of 5 M acetic acid. | The acetic acid prevents the precipitation of various anions such as borate, oxalate, phosphates etc. present in the sample. |
| Make up the volume with sulphate free water upto 50 ml. | Make up the volume with sulphate free water upto 50 ml. |
Hydrochloric acid helps to make solution acidic.
Potassium sulphate is used to increase the sensitivity of the test by giving ionic concentration in the reagent
Alcohol helps to prevent super saturation.
Also Read…. Limit test for Chloride
Conclusion
If opalescence produced in the standard is more than that of test, the sample complies the limit test of sulphate as per I.P. 1996.
Join Our WhatsApp Group to receive the latest updates like Pharma Job notifications, study materials, admission alerts, Pharma News, etc
Join Our Telegram Group to receive the latest updates like Pharma Job notifications, study materials, admission alerts, Pharma News, etc
Join Our Telegram Group to Download Free Books & Notes, Previous papers for D.Pharm, B.Pharm, M.Pharm, Drug Inspector & GPAT……….











I found this post on the limit test for sulphate incredibly insightful! The detailed explanation of the methodology and its significance in pharmaceutical chemistry really helped me understand its practical application. Thanks for sharing such valuable information!