PROPERTIES OF TRIACYLGLYCEROLS/FAT/OILS/Fatty acids
The Following properties of TGs/Fat/Oil are mainly used to detect adulteration/deterioration of oil/fat.
| S. No. | Properties | Reactant | Sample amount | To be determined | Use/ Application |
| 1 | Iodine value | I2 (No of gm of Iodine absorbed by) | 100 gm of Fat/oil | Iodine number i.e. indicate Unsaturation | Oil/Fat has specific Iodine no value, Use to check Adulteration |
| 2 | Saponification number | No of mg of KOH required to hydrolysis/saponify | 1 gm of fat/oil | Measures the average molecular size of Fatty acids/FA (Higher saponification no for fat/oil containing short chain FA | to check Adulteration |
| 3 | Acid number | No of mg of KOH required to neutralise free Fatty acid | Present in 1 gm of fat/oil | to determine free fatty acid (Rancidity liberate free acid) | To check oil decomposition/rancidity/adulteration |
| 4 | Reichert-Meissl (RM) number | No of ml of 0.1 N KOH or 1/10 N KOH required to neutralise soluble volatile fatty acid distilled | From 5 gm fat | to determine volatile fatty acid | Use to check purity of butter oil since butter oil contain volatile FA. |
| 5 | Hydroxyl value | No of mg of KOH required to neutralize acetic acid capable of combining by acetylation | With 1 gm fat/oil | ||
| 6 | Acetyl value | No of mg of KOH required to neutralize acetic acid | With 1 gm acetylated fat/oil is saponified | ||
| 7 | Ester value | No of mg of KOH require to combine with fatty acids | In 1 g oil/fat | Difference b/w saponification value & acid value is ester value. | |
| 8. | Polenski value | No of 0.1 N KOH require to neutralize water insoluble, steam-distilable acid liberated by hydrolysis of | 5 gm fat/oil |
1. Iodine Number:
It is defined as the grams (number) of iodine absorbed by 100 g of fat or oil. Iodine number is useful to know the relative unsaturation of fats, and is directly proportional to the content of unsaturated fatty acids. Thus lower is the iodine number, less is the degree of unsaturation.
2. Saponifiction:
The alkaline hydrolysis of triacylglycerols/fatty acid (esters)by alkali (KOH, NaOH) to produce glycerol (alcohol) and soaps is known as saponification. Lipids that contain fatty acid ester linkages can undergo hydrolysis. The mechanism of saponification is:
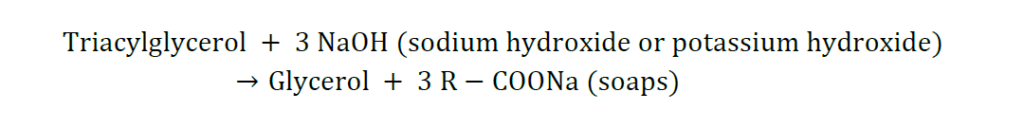
The reaction is used commercially to make soap, lubricants, and fire extinguishers.
3. Reichert-Meissl (RM) number:
It is defined as the number of ml 0.1 N KOH required to completely neutralize the soluble volatile fatty acids distilled from 5 g fat. RM number is useful in testing the purity of butter since it contains a good concentration of volatile fatty acids (butyric acid, caproic acid and caprylic acid). This is in contrast to other fats and oils which have a negligible amount of volatile fatty acids. Butter has a RM number in the range 25-30, while it is less than 1 for most other edible oils.
4. Acid number:
It is defined as the number of mg of KOH required to completely neutralize free fatty acids present in one gram fat or oil. Generally, refined oils should be free from any free fatty acids. Oils, on decomposition—due to chemical or bacterial contamination—yield free fatty acids. Therefore, oils with increased acid number are unsafe for human consumption.
5. Rancidity:
Unsaturated Fats and oils on exposure to air, moisture, bacteria etc. undergo rancidity (deterioration- unpleasant taste). This can be prevented by the addition of certain antioxidants (vitamin E, hydroquinone, gallic acid). To prevent rancidity of oil/fat, fat soluble anti-oxidant (vitamin E, hydroquinone, gallic acid) are added.
6. Hydroxyl value
No of mg of KOH required to neutralize acetic acid capable of combining by acetylation With 1 gm fat/oil.
7. Acetyl value
No of mg of KOH required to neutralize acetic acid With 1 gm acetylated fat/oil is saponified.
8. Ester value
No of mg of KOH require to combine with fatty acid in 1 gm oil/fat. Difference b/w saponification value & acid value is ester value.
Ester value = Saponification value – Acid value
9. Polenski value
No of 0.1 N KOH require to neutralize water insoluble, steam-distilable acid liberated by hydrolysis of 5 gm fat/oil sample.
MCQ practice
Q. 1: Iodine value of an oil sample is the measure of:
(a) Mean molecular weight of fatty acids
*(b) Unsaturated fatty acids
(c) Free fatty acids
(d) Unsaponifiable matter
Q.2- Reichert-Meissl (RM) number of an oil sample is the measure of:
(a) Mean molecular weight of fatty acids
(b) Unsaturated fatty acids
*(c) to determine volatile fatty acid
(d) Unsaponifiable matter
Q.3. Which of the following statement is correct –
[a] Ester value = Saponication value + Acid value
*[b] Ester value = Saponication value – Acid value
[c] Ester value = Saponication value × Acid value
[d] Ester value = Saponification value ÷ Acid value
Join WhatsApp channel to get latest Job notification, Study material, Previous paper, MCQ quiz, Admission alerts & News etc. for Pharmacy aspirants.
Subscribe our Telegram channel for Pharmacy Notes, MCQ Quiz, , Previous paper, Admission alerts & News etc. for Pharmacy professionals.
Join Telegram group for all Pharmacy books, Pharmacopoeia (IP, USP, BP), Pharmacy Notes, Previous Year Question papers in pdf format.