Pharmaceutical Syrup
Syrup
The syrup is a saturated or concentrated, viscous aqueous solution of sucrose/sugar substitute with or without flavor/medicinal substances in purified water.
Simple syrup contain 85% w/v (65% w/w); specific gravity 1.313 (USP) or 66.7% w/w as per Indian Pharmacopeia/BP.
| Simple syrup IP | Simple syrup USP |
|---|---|
| 66.7 w/w % solution of sugar/sucrose in purified water. | 85 % w/w solution of sugar/sucrose in purified water. |
| Prepared by hot process. | Prepared by cold process. |
| It can be checked during the process by using saccharometer | It cannot be checked during the process. |
| Invert sugar- Sucrose is heated during preparing → Invert sugar | Sucrose |
| More stable | Less stable |
Master Your Pharmacist Exam Preparation with The Pharmapedia’s Online Test Series and Previous Year Question Papers
Composition of Syrup
Most syrups contain the following components in addition to the purified water and drug(s):
- (a) Sugar, usually sucrose or other sugar substitutes are used to provide sweetness and viscosity, (Sugar-free alternative- Sorbitol, Saccharine, Aspartame)
- (b) Antimicrobial preservatives,
- (c) flavorants & colorants,
- (d) Syrups may also contain solubilizing agents, thickeners, or stabilizers.
Note:
Syrup may contain preservatives. Glycerin, methylparaben, benzoic acid, and sodium benzoate may be used to prevent bacterial and mold growth. Glycine, benzoic acid (0.1%–0.2%), sodium benzoate (0.1%– 0.2%), and various combinations of methylparaben, propylparaben, and butylparaben or alcohol are commonly used as antimicrobial preservatives.
Types of Syrup
1. Simple Syrup
When Purified Water alone is used in making the solution of sucrose, the preparation is known as “syrup,” or “simple syrup
or
Simple syrup contains only sucrose (sugar) & Purified water.
Example:
a. Simple Syrup IP
| Sucrose | 66.7 gm |
| Purified water q.s. | 100 gm |
b. Simple Syrup USP
| Sucrose | 85 gm |
| Purified water q.s. | 100 mL |
2. Medicated syrup
When Syrup contains medicinal substance is know as medicated syrup – cough syrup, Ginger syrup
Ginger syrup
| Strong Ginger tincture | 5 mL |
| Syrup q.s. | 100 mL |
3. Flavoured Syrup
Syrups containing flavoring agents but not medicinal substances are called flavored vehicles; Containing Aromatic/ Flavoured – Flavoured syrup
Example: Cherry & Raspberry syrup

Advantages of Syrup
- Act as Antioxidant- Retard oxidation because sugar partly hydrolyzed into dextrose & levulose (reducing sugary ) – So prevent decomposition of many substances – No preservative needed.
- Act as preservative- Exert high osmotic pressure – prevents the growth of MOs (Bacteria, Fungi, molds etc)
- Act as Palatable sweet – a vehicle for bitter / Nouseous substances.
- Prevent decomposition of many vegetable drugs.
Method of Preparation of Syrups
Syrups are prepared using one of four techniques:
- Solution with heat,
- Solution by agitation,
- Addition of sucrose to a liquid medication or flavored liquid
- Percolation.
1. Solution with Heat/Hot process
- This method is a suitable preparation method, if the constituents are not volatile or not degraded by heat.
- Purified water is heated to 80–85°C, and then removed from its heat source.
- Weight desired amount of Sucrose is added with vigorous agitation.
- Then, other required heat-stable components are added to the hot syrup, the mixture is allowed to cool, and its volume is adjusted to the proper level by the addition of purified water.
- In instances in which heat-labile agents or volatile substances, such as flavors and alcohol, are added, they are incorporated into the syrup after cooling to room temperature.
Example: Syrup IP, Acacia Syrup NF, Cocoa syrup NF, Tolu Syrup IP
Inversion of sugar
Invert sugar: When heat is used in the preparation of syrups, inversion of a slight portion of the sucrose (a disaccharide) into monosaccharides, dextrose (glucose), and fructose (levulose) by hydrolyzation process. This hydrolytic reaction is referred to as “inversion,” and the combination of the two monosaccharide products is “invert sugar.”
Sucrose solutions are dextrorotary, but, as hydrolysis proceeds, the optical rotation decreases and becomes negative when the reaction is complete. The rate of inversion is increased greatly by the presence of acids; the hydrogen ion acts as a catalyst in this hydrolytic reaction.
Fructose is responsible for the darkening of syrup.
Invert sugar is more readily fermentable than sucrose and tends to be darker in color. But, its two reducing sugars prevent the oxidation of other substances.
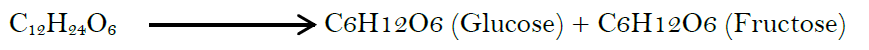
2. Solution by Agitation (without Heat)
This method is used for substances that degradation on heating or volatilize formulation constituents. i.e suitable for heat labile substances.
Sucrose & other ingredients are dissolved in purified water through agitation (without heat).
Example– Sulphate syrup
3. Addition of Sucrose to a Liquid Medication or Flavored Liquid:-
- This method is often used with fluidextracts or tinctures.
- Fluid extract or tinctures are added to a syrup.
- Addition of these may cause precipitation of alcohol soluble materials due to dilution.
- Example- Aromatic eridictyon syrup NF
4. Percolation (Cold process)
In the percolation method, either purified water or the medicinal component (dissolve in purified water) is passed slowly through a bed of crystalline sucrose, thus, dissolving it and forming a syrup.
- Sucrose is placed in suitable percolator.
- Purified water (with medicament) is allowed to slowly pass through sucrose
- Percolate may return back in necessary
- final volume is adjusted by purified water
Example: Ipecac syrup
Formulation of Syrup
1. Vehicle-
Purified water is used to prepare syrups.
2. Additives
a. Chemical Stabilizers
Glycerin, sorbitol & propylene glycol are addded in a small quantity to prevent Crystallization of Sucrose.
b. Colouring agents
Coal tar dyes like Amaranth, tartrazine & green S are added to syrup.
c. Flavouring agents
- Tincture- tincture, lemon & ginger
- Fruit juice- rasbberry juice, wild cherry
- Essence- Vanilla, orange
d. Preservatives
Benzoic acid & Its derivatives, Sod. benzoate, Methyl paraben etc
Storage of Syrup
Store in dried, well closed bottles in a coll dark place, below 25 oC.
- Syrup on Storage subject to Crystallisation which causes locking of Cap of the container. Glycerine, Sorbital & Propylene glycol is added in small quantity to the syrup to prevent crystallization of sucrose.
- Storage: completely filled & well-stoppered bottle and stored in a cool place (not exceeding 25-degree centigrade)
- Syrup may be dark color due to the fermentation of sugar.
Join WhatsApp channel to get latest Job notification, Study material, Previous paper, MCQ quiz, Admission alerts & News etc. for Pharmacy aspirants.
Subscribe our Telegram channel for Pharmacy Notes, MCQ Quiz, , Previous paper, Admission alerts & News etc. for Pharmacy professionals.
Join Telegram group for all Pharmacy books, Pharmacopoeia (IP, USP, BP), Pharmacy Notes, Previous Year Question papers in pdf format.











Thank you for the help, I truly appreciate