The Extraordinary Gazette was published by PCI on February 24, 2022. According to this Gazette of India, diploma in pharmacy students will have to pass the exit test in order to register as a pharmacist with the Pharmacy Council and get a retail drug licence after completion from the D.Pharmacy course from the session 2021–2022.
असाधारण राजपत्र 24 फरवरी, 2022 को पीसीआई द्वारा प्रकाशित किया गया था। भारत के इस राजपत्र के अनुसार, सत्र 2021-2022 से डी.फार्मेसी पाठ्यक्रम में डिप्लोमा करने वाले छात्रों को फार्मेसी काउंसिल के साथ फार्मासिस्ट के रूप में पंजीकरण करने और पूरा होने के बाद खुदरा दवा लाइसेंस प्राप्त करने के लिए एग्जिट टेस्ट पास करना होगा।
As per the current schedule, the Diploma in Pharmacy Exit Examination (DPEE) shall be conducted by the NBEMS biannually. The DPEE shall be conducted in March/April and September/October every year. The first session of DPEE shall be held in October 2024 as per schedule detailed under Important Dates and Scheme of Examination.
वर्तमान कार्यक्रम के अनुसार, डिप्लोमा इन फार्मेसी एग्जिट परीक्षा (DPEE) NBEMS द्वारा द्विवार्षिक रूप से आयोजित की जाएगी। DPEE हर साल मार्च/अप्रैल और सितंबर/अक्टूबर में आयोजित की जाएगी। DPEE का पहला सत्र महत्वपूर्ण तिथियों और परीक्षा योजना के तहत विस्तृत कार्यक्रम के अनुसार अक्टूबर 2024 में आयोजित किया जाएगा।
Examination Fee
| Examination Fee | GST | Total fee |
| 5000/- | 900/- | 5900/- |
Eligibility Criteria
- Candidates who have passed Diploma in Pharmacy course from an institution approved by the Pharmacy Council of India
- The result of final examination (D.Pharma Part-II) for the said Diploma in Pharmacy qualification should have been declared on or before 31st July 2024.
Exam pattern of Diploma in Pharmacy Exit Exam
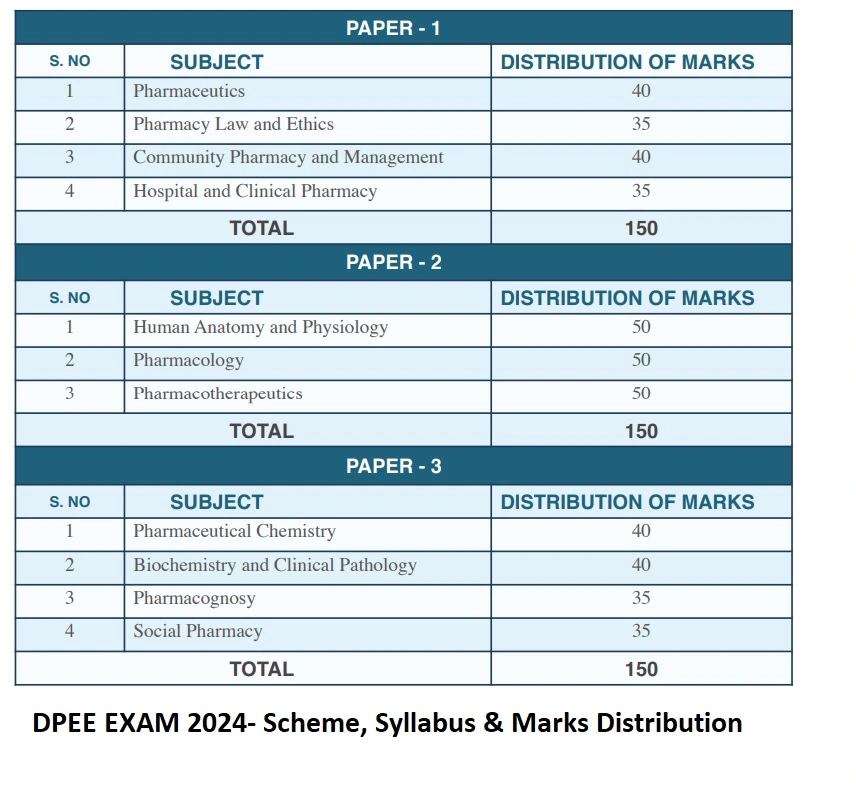
- There shall be Three paper of multiple choice questions in subjects/knowledge areas covered in the the syllabus as per the Diploma in Pharmacy Education Regulations 2020 (ER 2020) issued by Pharmacy Council of India
- These three paper i.e. Paper – 1, Paper – 2 & Paper – 3 shall be conducted on three consecutive days i.e. 3rd, 4th and 5th October 2024 respectively. Each paper shall be comprising of 150 questions to be attempted in 180 minutes.
- The Language of the EXIT exam will be English,
- The duration of exam will be 180 minutes.
- no negative marking
- Each question paper for DPEE will be divided into multiple time bound sections. For example, if there are 3-time bound sections* (Section A, B & C) in the question paper, each Section will have 50 questions and 60 minutes of time allotted for the section. Candidates would be restricted to proceed to the next section until completion of the allotted time of the previous section and candidates would not be allowed to review the questions/modify the responses of a section after the completion of the allotted time of that section. Questions of the next section will start automatically after the completion of the allotted time of the previous section.
| Description | Remarks |
|---|---|
| Question Paper | THREE MCQ type Papers |
| Duration of Exam | Each paper-180 minutes |
| Negative marking | No |
| Language of Paper | English only |
| Passing Marks | 50% in Each Paper |
| Attempt | Unlimited |
| Frequency of Exam | every six month () |
Passing Marks and attempts for EXIT EXAM
- There shall be no restriction on the number of attempts to appear in the examination.
- There is necessary to obtained 50 % marks in the paper to pass the exam
- After passing the EXIT exam, A certificate of eligibility for enrolment and practice shall be issued to the successful candidate which will be presented before the State Pharmacy Council for registration as a pharmacist.
SYLLABUS FOR D PHARMACY EXIT EXAM/डी.फार्मेसीएग्जिटपरीक्षाकेलिएसिलेबस | PCI
The Syllabus for the DPEE shall comprise of subjects /knowledge areas as per the Diploma in Pharmacy Education Regulations 2020 (ER 2020) issued by Pharmacy Council of India. An extract of the same is as follows:
1. Pharmaceutics
| S. No. | TOPICS |
| 1 | History of the profession of Pharmacy in India in relation to Pharmacy education, industry, pharmacy practice, and various professional associations. Pharmacy as a career Pharmacopoeia: Introduction to IP, BP, USP, NF and Extra Pharmacopoeia. Salient features of Indian Pharmacopoeia |
| 2 | Packaging materials: Types, selection criteria, advantages and disadvantages of glass, plastic, metal, rubber as packaging materials |
| 3 | Pharmaceutical aids: Organoleptic (Colouring, flavouring, and sweetening) agents Preservatives: Definition, types with examples and uses |
| 4 | Unit operations: Definition, objectives/applications, principles, construction, and workings of – 4.1:-Size reduction: Hammer mill and ball mill 4.2:- Size separation: Classification of powders according to IP, Cyclone separator, Sieves and standards of sieves 4.3:- Mixing: Double cone blender, Turbine mixer, Triple roller mill and Silverson mixer homogenizer 4.4:- Filtration: Theory of filtration, membrane filter and sintered glass filter 4.5:- Drying: working of fluidized in bed d er and process of freeze drying 4.6:- Extraction: Definition, Classification, method, and applications |
| 5 | 5.1:- Tablets – Coated and uncoated, various modified tablets (sustained release, extended-release, fast dissolving, multilayered, etc. 5.2:- Capsules – Hard and soft gelatine capsules 5.3:- Liquid oral preparations – Solution, syrup, elixir, emulsion, suspension, dry powder for reconstitution 5.4:- Topical preparations – Ointments, creams, pastes, gels, liniments and lotions, suppositories, and pessaries 8 Nasal reparations, Ear preparations 5.5:- Powders and granules – Insufflations, dusting powders, effervescent powders, and effervescent granules. 5.6:- Sterile formulations – Injectables, eye drops and eye ointments 5.7:- Immunological products: Sera, vaccines, toxoids, and their manufacturing methods. |
| 6 | Basic structure, layout, sections, and activities of pharmaceutical manufacturing plants Quality control and quality assurance: Definition and concepts of quality control and quality assurance, current good manufacturing practice (cGMP), Introduction to the concept of calibration and validation |
| 7 | Novel drug delivery systems: Introduction, Classification with examples, advantages, and challenges |
2. Pharmacology
| S. No. | TOPIC | |
| 1 | GENERAL PHARMACOLOGY 1.1:- Introduction and scope of Pharmacology 1.2:-Various routes of drug administration – advantages and disadvantages 1.3:-Drug absorption – definition, types, factors affecting drug absorption 1.4:-Bioavailability and the factors affecting bioavailability 1.5: Drug distribution – definition, factors affecting drug distribution 1.6: Biotransformation of drugs – Definition, types of biotransformation reactions, factors influencing drug metabolisms 1.7:-Excretion of drugs – Definition, routes of drug excretion 1.8:-General mechanisms of drug action and factors modifying drug action | |
| 2 | DRUGS ACTING ON THE PERIPHERAL NERVOUS SYSTEM 2.1:-Steps involved in neurohumoral transmission 2.2:-Definition, classification, pharmacological actions, dose, indications, and contraindications of 2.2.1:- Cholinergic drugs & Anti-Cholinergic drugs 2.2.2:- Adrenergic drugs & Anti-adrenergic drugs 2.2.3:- Neuromuscular blocking agents 2.2.4:- Drugs used in Myasthenia gravis 2.2.5:- Local anesthetic agents 2.2.6:- Non-Steroidal Anti-Inflammatory drugs (NSAIDs) | |
| 3 | Drugs Acting on the Eye:- Definition, classification, pharmacological actions, dose, indications and contraindications of- 3.1:-Miotics 3.2:-Mydriatics 3.3:-Drugs used in Glaucoma | |
| 4 | DRUGS ACTING ON THE CENTRAL NERVOUS SYSTEM:- Definition, classification, pharmacological actions, dose, indications, and contraindications of- 4.1:- General anesthetics 4.2:- Hypnotics and sedatives 4.3:- Anti-Convulsant drugs 4.4:- Anti-anxiety drugs 4.5:- Anti-depressant drugs 4.6:- Anti-psychotics 4.7:- Nootropic agents 4.8:- Centrally actin muscle relaxants 4.9:- Opioid analgesics | |
| 5 | Drugs Acting on the Cardiovascular System- Definition, classification, pharmacological actions, dose, indications, and contraindications of:- 5.1:- Anti-hypertensive drugs 5.2:- Anti-anginal drugs 5.3:- Anti-arrhythmic drugs 5.4:- Drugs used in atherosclerosis 5.5:- Congestive heart failure 5.6:- Drug therapy for shock | |
| 6 | Drugs Acting on Blood and Blood Forming Organs– Definition, classification, pharmacological actions, dose, indications, and contraindications of:- 6.1:- Hematinic agents 6.2:- Anti-coagulants 6.3:- Anti-platelet agents 6.4:- Thrombolytic drugs | |
| 7 | Definition, classification, pharmacological actions, dose, indications, and contraindications of:- 7.1:- Bronchodilators 7.2:- Mucolytic agents 7.3:- Expectorants 7.4:- Anti-tussive agents | |
| 8 | Drugs Acting on the Gastro Intestinal Tract– Definition, classification, pharmacological actions, dose, indications, and contraindications of- 8.1:- Anti-ulcer drugs 8.2:- Anti-emetics 8.3:- Laxatives and purgatives 8.4:- Anti-diarrheal drugs | |
| 9 | Drugs Acting on the Kidney– Definition, classification, pharmacological actions, dose, indications, and contraindications of:- 9.1:- Diuretics 9.2:- Anti-Diuretics | |
| 10. | Hormones and Hormone Antagonists:- Physiological and pathological role and clinical uses of 10.1:- Thyroid hormones 10.2:- Anti-thyroid drugs 10.3:- Parathormone 10.4:- Calcitonin 10.5:- Vitamin D 10.6:- Insulin 10.7:- Oral hypoglycemic agents 10.8:- Estrogen 10.9:- Progesterone 10.10:- Oxytocin 10.11:- Corticosteroids | |
| 11 | Autocoids 11.1:- Physiological role of Histamine, 5 HT and Prostaglandins 11.2:- Classification, clinical uses, and adverse effects of antihistamines and 5HT antagonists | |
| 12 | Chemotherapeutic Agents: Introduction, basic principles of chemotherapy of infections, infestations and neoplastic diseases, Classification, dose, indication and contraindications of drugs belonging in to following classes:- 12.1:- Penicillins 12.2:- Cephalosporins 12.3:- Aminoglycosides 12.4:- Fluoroquinolones 12.5:- Macrolides 12.6:- Tetracyclines 12.7:- Sulphonamides 12.8:- Anti-tubercular drugs 12.9:- Anti-fungal drugs 12.10:- Anti-viral drugs 12.11:- Anti-amoebic agents 12.12:- Anthelmintics 12.13:- Anti-malarial agents 12.14:- Anti-neoplastic agents | |
| 13 | Biologicals Definition, types, and indications of biological agents with examples | |
3. Pharmacognosy
| S. No. | TOPIC |
| 1. | Definition, history, present status, and scope of Pharmacognosy |
| 2. | Classification of drugs: 2.1:- Alphabetical 2.2:- Taxonomical 2.3:- Morphological 2.4:- Pharmacological 2.5:- Chemical 2.6:- Chemo-taxonomical |
| 3. | Quality control of crude drugs: 3.1:- Different methods of adulteration of crude drugs 3.2:- Evaluation of crude drugs |
| 4. | Brief outline of occurrence, distribution, isolation, identification tests, therapeutic activity, and pharmaceutical applications of alkaloids, terpenoids, glycosides, volatile oils, tannins, and resins |
| 5. | Biological source, chemical constituents, and therapeutic efficacy of the following categories of crude drugs:- 5.1:- Laxatives:-Aloe, Castor oil, Ispaghula, Senna 5.2:- Cardiotonic:- Digitalis, Arjuna 5.3:- Carminatives and G.I. regulators: Coriander, Fennel, Cardamom, Ginger, Clove, Black Pepper, Asafoetida, Nutmeg, Cinnamon 5.4:- Astringents:- Myrobalan, Black Catechu, Pale Catechu 5.5:- Drugs acting on nervous system:- Hyoscyamus, Belladonna, Ephedra, Opium, Tea leaves, Coffee seeds, Coca 5.6:- Anti-hypertensive– Rauwolfia 5.7:- Anti-tussive -Vasaka, Tolu Balsam 5.8:- Anti-rheumatics– Colchicum seed 5.9:- Anti-tumour– Vinca, Podophyllum 5.10:- Antidiabetics– Pterocarpus, Gymnema 5.11:- Diuretics– Gokhru, Punarnava 5.12:- Anti-dysenteric– Ipecacuanha 5.13:- Antiseptics and disinfectants– Benzoin, Myrrh, Neem, Turmeric 5.14:- Antimalarials– Cinchona, Artemisia 5.15:- Vitamins– Cod liver oil Shark liver oil 5.16:- Oxytocic– Ergot 5.17:- Enzymes– Papaya, Diastase, Pancreatin, Yeast 5.18:- Pharmaceutical Aids– Kaolin, Lanolin, Beeswax, Acacia, Tragacanth, Sodium alginate, Agar, Guar gum, Gelatin 5.19:- Miscellaneous– Squill, Galls, Ashwagandha, Tulsi, Guggul |
| 6. | Plant fibers used as surgical dressings: 6.1:- Cotton, silk, wool, and regenerated fibers 6.2:- Sutures – Surgical Catgut and Ligatures |
| 7. | 7.1:- Basic principles involved in the traditional systems of medicine like: Ayurveda, Siddha, Unani, and Homeopathy 7.2:-Method of preparation of Ayurvedic formulations like: Arista, Asava, Gutika, Taila, Churna, Lehya and Bhasma |
| 8. | Role of medicinal and aromatic plants in national economy and their export potential |
| 9. | Herbs as health food: Brief introduction and therapeutic applications of: 9.1:- Nutraceuticals 9.2:- Antioxidants 9.3:- Pro-biotics 9.4:-Pre-biotics 9.5:- Dietary fibers 9.6:- Omega 3-fatty acids, 9.7:- Spirulina 9.8:- Carotenoids 9.9:- Soya and 9.10:- Garlic |
| 10. | Introduction to herbal formulations |
| 11. | Herbal cosmetics: Sources, chemical constituents, commercial preparations, therapeutic and cosmetic uses of: 11.1:- Aloe vera gel 11.2:- Almond oil 11.3:- Lavender oil 11.4:- Olive oil 11.5:- Rosemary oil 11.6:- Sandal Wood oil |
| 12. | Phytochemical investigation of drugs |
4. Pharmaceutical chemistry
| S. No. | TOPICS |
| 1. | 1.1:- Introduction to Pharmaceutical chemistry: Scope and objectives 1.2:- Sources and types of errors: Accuracy, precision, significant figures 1.3:- Impurities in Pharmaceuticals: Source and effect of impurities in Pharmacopeial substances, importance of limit test„ Principle and procedures of Limit tests for chlorides, sulphates, iron, heavy metals and arsenic, |
| 2. | 2.1:- Volumetric analysis: Fundamentals of volumetric analysis, Acid-base titration, non-aqueous titration, precipitation titration, complexometric titration, redox titration 2.2:- Gravimetric analysis: Principle and method. |
| 3. | Inorganic Pharmaceuticals: Pharmaceutical formulations, market preparations, storage conditions and uses of- Haematinics – Ferrous sulphate, Ferrous fumarate, Ferric ammonium citrate, Ferrous ascorbate, Carbonyl iron.Gastro-intestinal Agents – Antacids: Aluminium hydroxide gel, Magnesium hydroxide, Magaldrate, Sodium bicarbonate, Calcium Carbonate, Acidifying agents, Adsorbents, Protectives, CatharticsTopical agents – Silver Nitrate, Ionic Silver, Chlorhexidine Gluconate, Hydrogen peroxide, Boric acid, Bleaching powder, Potassium permanganateDental products – Calcium carbonate, Sodium fluoride, Denture cleaners, Denture adhesives, Mouth washesMedicinal gases – Carbon dioxide, nitrous oxide, oxygen |
| 4. | Introduction to nomenclature of organic chemical systems with particular reference to heterocyclic compounds containing up to Three rings |
| Study of the following category of medicinal compounds with respect to classification, chemical name, chemical structure (compounds marked with*) uses, stability and storage conditions, different of formulations and their popular brand names:- | |
| 5. | DRUGS ACTING ON CENTRAL NERVOUS SYSTEM 5.1:- Anesthetics: Thiopental Sodium*, Ketamine Hydrochloride*, Propofol 5.2:- Sedatives and Hypnotics: Diazepam*, Alprazolam*, Nitrazepam, Phenobarbital* 5.3:- Antipsychotics: Chlorpromazine Hydrochloride*, Haloperidol*, Risperidone*, Sulpiride*, Olanzapine, Quetiapine, Lurasidone 5.4:- Anticonvulsants: Phenytoin*j Carbamazepine*, Clonazepam, Valproic Acid*, Gabapentin*, Topiramate, Vigabatrin, Lamotrigine 5.5:- Anti-Depressants Amitriptyline Hydrochloride*, Imipramine Hydrochloride*, Fluoxetine*, Venlafaxine, Duloxetine, Sertraline, Citalopram, Escitalopram, Fluvoxamine, Paroxetine |
| 6. | DRUGS ACTING ON AUTONOMIC NERVOUS SYSTEM 6.1:- Sympathomimetic Agents: Direct Acting: Nor Epinephrine*, Epinephrine, Phenylephrine, Dopamine*, Terbutaline, Salbutamol (Albuterol), Naphazoline*, Tetrahydrozoline. 6.1.1:- Indirect Acting Agents: Hydroxy Amphetamine, Pseudoephedrine. Agents With Mixed Mechanism: Ephedrine, Metaraminol 6.2:- Adrenergic Antagonists: Alpha Adrenergic Blockers: Tojazoline, Phentolamine, Phenoxybenzamine, Prazosin. Beta Adrenergic Blockers: Propranolol*, Atenolol*, Carvedilol 6.3:- Cholinergic Drugs and Related Agents: Direct Acting Agents: Acetylcholine*, Carbachol, And Pilocarpine. Cholinesterase Inhibitors: Neostigmine*, Edrophonium Chloride, Tacrine Hydrochloride, Pralidoxime Chloride, Echothiopate Iodide 6.4:- Cholinergic Blocking Agents: Atropine Sulphate*, Ipratropium Bromide 6.5:- Synthetic Cholinergic Blocking Agents: Tropicamide, Cyclopentolate Hydrochloride, Clidinium Bromide, Dicyclomine Hydrochloride |
| 7. | DRUGS ACTING ON CARDIOVASCULAR SYSTEM 7.1:- Anti-Arrhythmic Drugs: Quinidine Sulphate, Procainamide Hydrochloride, Verapamil, Phenytoin Sodium*, Lidocaine Hydrochloride, Lorcainide Hydrochloride, Amiodarone and Sotalol 7.2:- Anti-Hypertensive Agents: Propranolol*, Captopril*, Ramipril, Methyldopate Hydrochloride, Clonidine Hydrochloride, Hydralazine Hydrochloride, Nifedipine 7.3:- Antianginal Agents: Isosorbide Dinitrate |
| 8. | Diuretics: Acetazolamide, Frusemide*, Bumetanide, Chlorthalidone Benzthiazide, Metolazone, Xipamide, Spironolactone |
| 9. | Hypoglycemic Agents: Insulin and Its Preparations, Metformin*, Glibenclamide*, Glimepiride, Pioglitazone, Repaglinide, Gliflozins, Gliptins |
| 10. | 10.1:- Analgesic And Anti-Inflammatory Agents: Morphine Analogues, Narcotic Antagonists 10.2:- Nonsteroidal Anti-Inflammatory Agents (NSAIDs) – Aspirin*, Diclofenac, Ibuprofen*, Piroxicam, Celecoxib, Mefenamic Acid, Paracetamol*, Aceclofenac |
| 11. | ANTI-INFECTIVE AGENTS 11.1:- Antifungal Agents: Amphotericin-B, Griseofulvin, Miconazole, Ketoconazole Itraconazole, Fluconazole*, Naftifine Hydrochloride 11.2:- Urinary Tract Anti-Infective Agents: Norfloxacin, Ciprofloxacin, Ofloxacin” Moxifloxacin 11.3:- Anti-Tubercular Agents: INH*, Ethambutol, Para Amino Salicylic Acid. Pyrazinamide, Rifampicin, Bedaquiline, Delamanid. Pretomanid* 11.4:- Antiviral Agents: Amantadine Hydrochloride, Idoxuridine, Acyclovir”, Foscarnet, Zidovudine, Ribavirin, Remdesivir. Favipiravir 11.5:- Antimalarials: Quinine Sulphate, Chloroquine Phosphate% Primaquine Phosphate, Mefloquine*, Cycloguanil, Pyrimethamine, Artemisinin 11.6:- Sulfonamides: Sulfanilamide, Sulfadiazine, Sulfamethoxazole, Sulfacetamide*, Mafenide Acetate, Cotrimoxazole, Dapsone* |
| 12. | Antibiotics: Penicillin G, Amoxicillin, Cloxacillin, Streptomycin Tetracyclines: Doxycycline, Minocycline Macrolides: Erythromycin, Azithromycin, Miscellaneous: Chloramphenicol* Clindamycin |
| 13. | Anti-Neoplastic Agents: Cyclophosphamide% Busu!fan, Mercaptopurine, Fluorouracil*, Methotrexate, Dactinomycin, Doxorubicin Hydrochloride, Vinblastine Sulphate, Cisplatin*, Dromostanolone Propionate |
5. Biochemistry& Clinical Pathology
| S. No. | TOPIC |
| 1. | Introduction to biochemistry: scope of biochemistry in pharmacy; cell and its Biochemical organization |
| 2. | CARBOHYDRATES 2.1:- Definition, classification with examples, chemical properties 2.2:- Monosaccharides – Structure of glucose, fructose, and galactose 2.3:- Disaccharides – structure of maltose, lactose, and sucrose 2.4:- Polysaccharides – chemical nature of starch and glycogen 2.5:- Qualitative tests and biological role of carbohydrates |
| 3. | PROTEINS 3.1:- Definition, classification of proteins based on composition and solubility with examples 3.2:- Definition, classification of amino acids based on chemical nature and nutritional requirements with examples 3.2:- Structure of proteins (four levels of organization of protein structure) 3.4:- Qualitative tests and biological role of proteins and amino acids 3.5:- Diseases related to malnutrition of proteins |
| 4. | LIPIDS 4.1:- Definition, classification with examples 4.2:- Structure and properties of triglycerides (oils and fats) 4.3:- Fatty acid classification – Based on chemical and nutritional requirements with examples 4.4:- Structure and functions of cholesterol in the body 4.5:- Lipoproteins – types, composition, and functions in the body 4.6:- Qualitative tests and functions of lipids |
| 5. | NUCLEIC ACIDS 5.1:- Definition, purine, and pyrimidine bases Components of nucleosides and nucleotides with examples 5.2:- Structure of DNA (Watson and Crick model), RNA and their functions |
| 6. | ENZYMES 6.1:- Definition, properties and lUB and MB classification 6.2:- Factors affecting enzyme activity 6.3:- Mechanism of action of enzymes, Enzyme inhibitors 6.4:- Therapeutic and pharmaceutical importance of enzymes |
| 7. | VITAMINS 7.1:- Definition and classification with examples 7.2:- Sources, chemical nature, functions, coenzyme form, recommended dietary requirements 7.3:- Deficiency diseases of fat-and water-soluble vitamins |
| METABOLISM (Study of cycle/pathways without chemical structures) 8.1:- Metabolism of Carbohydrates: Glycolysis, TCA cycle and glycogen metabolism, regulation of blood glucose level. Diseases related to abnormal metabolism of Carbohydrates 8.2:- Metabolism of lipids: Lipolysis, ß-oxidation of Fatty acid (Palmitic acid) ketogenesis and ketolysis. Diseases related to abnormal metabolism of lipids such as Ketoacidosis, Fatty liver, Hypercholesterolemia 8.3:- Metabolism of Amino acids (Proteins): General reactions of amino acids and its significance— Transamination, deamination, Urea cycle and decarboxylation. 8.4:- Diseases related to abnormal metabolism of amino acids, Disorders of ammonia metabolism, phenylketonuria, alkaptonuria and Jaundice. 8.5:- Biological oxidation: Electron transport chain and Oxidative phosphorylation | |
| MINERALS:- Types, Functions, Deficiency diseases, recommended dietary requirements | |
| 10. | WATER AND ELECTROLYTES 10.1:- Distribution, functions of water in the body 10.2:- Water turnover and balance 10.3:- Electrolytes composition of the body fluids, Dietary intake of electrolyte and Electrolyte balance 10.4:- Dehydration, causes of dehydration and oral rehydration therapy |
| 11. | Introduction to Biotechnology |
| 12. | ORGAN FUNCTION TESTS 12.1:- Functions of kidney and routinely performed tests to assess the functions of kidney and their clinical significances 12.2:- Functions of liver and routinely performed tests to assess the functions of liver and their clinical significances 12.3:- Lipid profiles tests and its clinical significances |
| 13. | INTRODUCTION TO PATHOLOGY OF BLOOD AND URINE 13.1:- Lymphocytes and Platelets, their role in health and disease 13.2:- Erythrocytes – Abnormal cells and their significance 13.3:- Normal and Abnormal constituents of Urine and their significance |
6. Pharmacy laws & Ethics/ Pharmaceutical Jurisprudence
| S. No. | TOPIC |
| 1. | General Principles of Law, History and various Acts related to Drugs and Pharmacy profession |
| 2. | 2.1:- Pharmacy Act-1948 and Rules: Objectives, Definitions, Pharmacy Council of India; its constitution and functions, Education Regulations, State and Joint state pharmacy councils, Registration of Pharmacists, Offences and Penalties. 2.2:- Pharmacy Practice Regulations 2015 |
| Drugs and Cosmetics Act 1940 and Rules 1945 and New Amendments 3.1:- Objectives, Definitions, Legal definitions of schedules to the Drug & Cosmetic Act and Rules 3.2:- Import of drugs – Classes of drugs and cosmetics prohibited from import, Import under license or permit. 3.3:- Manufacture of drugs – Prohibition of manufacture and sale of certain drugs, Conditions for grant of license and conditions of license for manufacture of drugs, Manufacture of drugs for test, examination and analysis, manufacture of new drug, loan license and repacking license. 3.4:- Study of schedule C and C 1, G, H, HI, K, P, M, N, and X. 3.5:- Sale of Drugs – Wholesale, Retail sale and Restricted license, Records to be kept in a pharmacy Drugs Prohibited for manufacture and sale in India 3.6:- Administration of the Act and Rules – -Drugs Technical Advisory Board -Central Drugs Laboratory -Drugs Consultative Committee -Government analysts -Licensing authorities, controlling authorities &Drug Inspectors | |
| 4. | Narcotic Drugs and Psychotropic Substances Act 1985 and Rules Objectives, Definitions, Authorities and Officers, Prohibition, Control and Regulation, Offences and Penalties |
| 5. | Drugs and Magic Remedies (Objectionable Advertisements) Act 1954 Objectives, Definitions, Prohibition of certain advertisements, Classes of Exempted advertisements, Offences and Penalties |
| 6. | Prevention of Cruel to Animals Act-1960: 6.1:- Objectives, Definitions 6.2:- CPCSEA – brief overview, Institutional Animal Ethics Committee, Breeding and Stocking of Animals, Performance of Experiments, Transfer and Acquisition of animals for experiment, Records, Power to suspend or revoke registration 6.3:- Offences and Penalties |
| 7 | Poisons Act-1919: Introduction, objective, definition, possession, possession for sales and sale of any poison, import of poisons |
| FSSAI (Food Safety and Standards Authority of India) Act and Rules: Brief overview and aspects related to manufacture, storage, sale, and labelling of Food Supplements | |
| 9. | 9.1:- National Pharmaceutical Pricing Authority: Drugs Price Control Order (DPCO) -2013:- Objectives, Definitions, Sale prices of bulk drugs, Retail price of formulations, Retail price and ceiling price of scheduled formulations 9.2:- Pharmaceutical Policy 2002 9.3:- National List of Essential Medicines (NLEM) |
| 10. | Code of Pharmaceutical Ethics: Definition, ethical principles, ethical problem solving, registration, code of ethics for Pharmacist in relation to his job, trade, medical profession and his profession, Pharmacist’s oath. |
| 11. | Medical Termination of Pregnancy Act and Rules – basic understanding, salient features, and Amendments |
| 12. | Role of all the government pharma regulator bodies:- 12.1:- Central Drugs Standards Control Organization (CDSCO) 12.2:- Indian Pharmacopoeia Commission (IPC) |
| 13. | Good Regulatory practices (documentation, licenses, renewals, e-governance) in Community Pharmacy, Hospital pharmacy, Pharma Manufacturing, Wholesale business, inspections, import, export of drugs and medical devices |
| 14. | Introduction to BCS system of classification Basic concepts of Clinical Trials ANDA, NDA, New Drug development, New Drugs and Clinical Trials Rules, 2019 Brand v/s Generic, Trade name concept Introduction to Patent Law and Intellectual Property Rights, Emergency Use Authorization |
| 15. | Blood bank – Basic requirements and functions |
| 16. | Clinical Establishment Act and Rules – Aspects related to Pharmacy only |
| 17. | Biomedical Waste Management Rules 2016 – Basic aspects, and aspects related to pharma manufacture to disposal of pharma / medical waste at homes, pharmacies, and hospitals |
| 18. | Bioethics – Basic concepts, history and principles. Brief overview of ICMRs National Ethical Guidelines for Biomedical and Health Research involving human participants |
| 19. | Introduction to the Consumer Protection Act |
| 20. | Introduction to the Disaster Management Act |
| 21. | Medical Devices – Categorization, basic aspects related to manufacture and sale |
7. Hospital & Clinical Pharmacy
| S. No. | TOPIC |
| 1. | HOSPITAL PHARMACY 1.1:- Definition, scope, national and international scenario 1.2:- Organisational structure 1.3:- Professional responsibilities, Qualification and experience requirements, job specifications, work load requirements and inter professional relationships 1.4:- Good Pharmacy Practice (GPP) in hospital 1.5:- Hospital Pharmacy Standards (FIP Basel Statements, AHSP) 1.6:- Introduction to NAQS guidelines and NABH Accreditation and Role of Pharmacists |
| 2. | DIFFERENT COMMITTEES IN THE HOSPITAL 2.1:- Pharmacy and Therapeutics Committee – Objectives, Composition, and functions 2.2:- Hospital Formulary – Definition, procedure for development and use of hospital formulary 2.3:- Infection Control Committee – Role of Pharmacist in preventing Antimicrobial Resistance |
| 3. | SUPPLY CHAIN AND INVENTORY CONTROL 3.1:- Preparation of Drug lists – High Risk drugs, Emergency drugs, Schedule HI drugs, NDPS drugs, reserved antibiotics 3.2:- Procedures of Drug Purchases – Drug selection, short term, long term, and tender/e-tender process, quotations, etc. 3.3:- Inventory control techniques: Economic Order Quantity, Reorder Quantity Level, Inventory Turnover etc. 3.4:- Inventory Management of Central Drug Store – Storage conditions, Methods of storage, Distribution, Maintaining Cold Chain, Devices used for cold storage (Refrigerator, ILR, Walk-in-Cold rooms) 3.4:- FEFO, FIFO methods 3.5:- Expiry drug removal and handling, and disposal. Disposal of Narcotics, cytotoxic drugs 3.6:- Documentation – purchase and inventory |
| 4. | DRUG DISTRIBUTION 4.1:- Drug distribution (in- patients and out – patients) – Definition, advantages and disadvantages of individual prescription order method, Floor Stock Method, Unit Dose Drug Distribution Method, Drug Basket Method. 4.2:- Distribution of drugs to ICCU/ICU/NlCU/Emergency wards. 4.3:- Automated drug dispensing systems and devices 4.4:- Distribution of Narcotic and Psychotropic substances and their storage |
| 5. | Compounding in Hospitals. Bulk compounding, IV admixture services and incompatibilities, Total parenteral nutrition |
| 6. | Radio Pharmaceuticals – Storage, dispensing and disposal of radio pharmaceuticals |
| 7. | Application of computers in Hospital Pharmacy Practice, Electronic health records, Software’s used in hospital pharmacy |
| 8. | 8.1:- Clinical Pharmacy: Definition, scope, and development – in India and other countries 8.2:- Technical definitions, common terminologies used in clinical settings and their significance such as Paediatrics, Geriatric, Anti-natal Care, Post-natal Care, etc 8.3:- Daily activities of clinical pharmacists: Definition, goal, and procedure of – Ward round participation -Medication history -Treatment Chart Review -Patient counselling -Adverse drug reaction monitoring -Drug information & poisons information -Interprofessional collaboration 8.4:- Pharmaceutical care:- Definition, classification of drug related problems. Principles & procedure to provide pharmaceutical care 8.5:- Medication Therapy Management, Home Medication Review |
| 9. | Clinical laboratory tests used in the evaluation of disease states – significance and interpretation of test results 9.1:-Haematological, Liver function, Renal function, thyroid function tests, 9.2:-Tests associated with cardiac disorders, 9.3:-Fluid and electrolyte balance, 9.4:-Pulmonary Function Tests |
| 10. | 10.1:-Poisoning: Types of poisoning: Clinical manifestations and Antidotes 10.2:-Drugs & Poison Information Centre and their services – Definition, Requirements, Information resources with examples, and their advantages and disadvantages |
| 11. | Pharmacovigilance:-Definition, aim and scope, Overview of Pharmacovigilance |
| 12. | 12.1:-Medication errors: Definition, types, consequences, and strategies to minimize medication errors, LASA drugs and Tallman lettering as per ISMP 12.2:-Drug Interactions: Definition, types, clinical significance of drug interactions |
Download Syllabus pdf
Join WhatsApp channel to get latest Job notification, Study material, Previous paper, MCQ quiz, Admission alerts & News etc. for Pharmacy aspirants.
Subscribe our Telegram channel for Pharmacy Notes, MCQ Quiz, , Previous paper, Admission alerts & News etc. for Pharmacy professionals.
Join Telegram group for all Pharmacy books, Pharmacopoeia (IP, USP, BP), Pharmacy Notes, Previous Year Question papers in pdf format.


